SERVICES
I. Assessment of Chemicals and Products
REACH-Registration
REACH-elements as required by the European Chemicals Agency (ECHA):
- Substance Identity
-
Registration Dossier
Technical Dossier
Chemical Safety Report (CSR)
-
Evaluation (of testing proposals and dossiers)
-
Authorisation (only substances of very high concern)
BTO offers support on identification of a given chemical substance and preparation of the registration dossier, in particular the chemical safety report (CSR) consisting on:
- Hazard Assessment
-
Exposure Estimation
-
Risk Characterisation
Hazard assessment as a core element of REACH depends on an tailored strategy for toxicological testing.
Testing Strategy
BTO prepares a testing strategy individually for a certain chemical or product according to the respective legal directive. All information available will be considered to indentify those tests, which can be waived for scientific or technical reasons.

Tailored testing strategies could minimise or avoid toxicological testing and, therefore, reduce costs markedly.
Alternatives to Animal Testing
Tests, which nessessarily must be conducted, are conventionally animal experi-ments. This kind of experimentation is extremely time consuming and labour intensive and, therefore, quite expensive. For this reason and for ethical grounds, BTO focusses on the reduction and replacement of animal experiments by the implementation of alternative methods.
Use of alternative test methods lead to cost effectivness and demontrates a high ethical sense of responsibility.
Assessment of Toxicological Tests
After finishing experimental work in the laboratories, the final test report needs to be assessed. The assessment leads to a classification of the test chemical as toxic (resp. non-toxic) or requires further testing. BTO is highly experienced in assessing all toxicological endpoints, in particular toxicity to reproduction, genetic toxicity and inhalation toxicity. The result will possibly be classification and labelling of a chemical or product for every single endpoint (REACH GHS). A summary of the single assessments ends up in the overall toxicological pattern, which is crucial for commercial exploitation of the chemical or product.
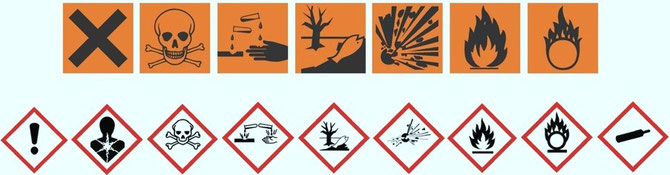
Marketability of a chemical or a product depends on its classification and labelling (CLP).
II. Development of Alternatives to Animal Experiments
Validation and Implementation
BTO is involved in the devepolment of new testing methods with special emphasis on acute toxicity after inhalation and on toxicity to reproduction. BTO supports the worldwide acceptance of alternatives by including them into guideline collections, e.g. that of the OECD.
Steps towards a new test method according to a Period (approx.)
harmonised OECD validation concept (1996)
=====================================================
5. Regulatory implementation 10 years
-
Establishment of a directive (e.g. OECD)
4. Validation 3 years
-
ESAC statement
- Peer review
- Relevance und reliability
- Ring trial
3. Pre-Validation 2 years
-
Inter-laboratory comparison
- Improvement of the test protocol
- Improvement of the predictions model
2. Test development 5 years
-
Prediction model
- Test protocol
1. Basic research 3 years
-
Scientific basis
- Applicability domain
New test methods are not a scientific purpose in themselves, but must be applied to regulatory toxocology to ensure the saftey of chemicals and products.
 Berlin Toxicology Office
(BTO)
Berlin Toxicology Office
(BTO)

